|
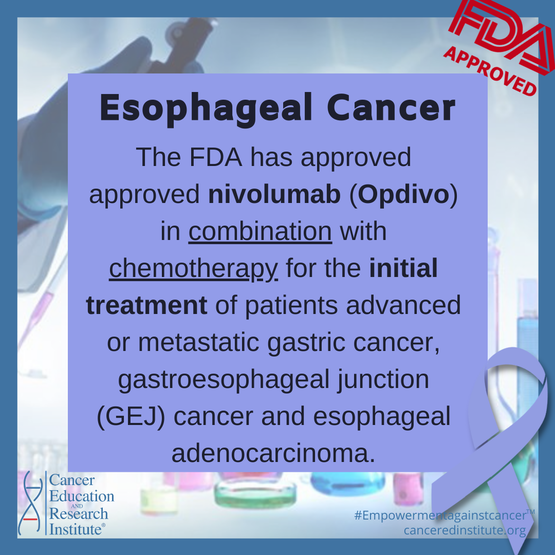
Today, on April 16, 2021, the Food and Drug Administration (FDA) approved nivolumab (Opdivo, Bristol-Myers Squibb Company) in combination with certain chemotherapy for patients with for the initial treatment of patients with advanced or metastatic gastric cancer, gastroesophageal junction (GEJ) cancer and esophageal adenocarcinoma.
This is the first FDA-approved immunotherapy for the first-line treatment of gastric cancer.
Reference:
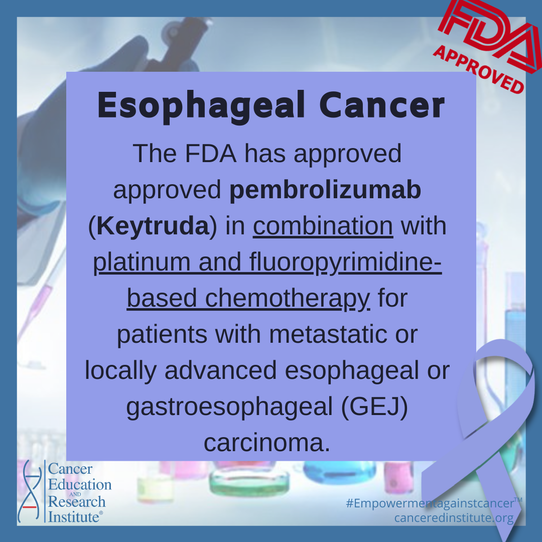
On March 22, 2021, the Food and Drug Administration (FDA) approved pembrolizumab (Keytruda, Merck Sharp & Dohme Corp.) in combination with platinum and fluoropyrimidine-based chemotherapy for patients with metastatic or locally advanced esophageal or gastroesophageal (GEJ) (tumors with epicenter 1 to 5 centimeters above the gastroesophageal junction) carcinoma who are not candidates for surgical resection or definitive chemoradiation.
Reference:
This is our long-time game we've created, the CERI Cancer Quiz Game™!
Test your cancer knowledge with CERI - take today's quiz, share with you friends! Every Wednesday! You can find the correct answer in two of our videos on our Youtube channel: COME BACK EVERY WEDNESDAY FOR OUR 'QUESTION OF THE DAY' QUIZ! By Ayguen Sahin, MSc, PhD - Cancer Education and Research Institute In some cancers, cancer cells can invade in along nerves causing an aggressive perineural invasion (PNI, the invasion of cancer to the space surrounding a nerve), which leads to pain and paralysis, and reduced survival.
A study led by Deborde and colleagues showed that cancer cells exploit normal Schwann cell nerve repair programs to promote perineural invasion. This research study observed these results in:
Schwann cells are a type of glial cells, which surrounds neurons and play an essential role in the fuction and survival of the neurons. Cancer cells formed protrusions and migration toward Schwann cells through a protein called NCAM1 (Neural Adhesion Molecule 1), which is correlated with the presence of perineural invasion. Perineural invasion (PNI) is also often seen in:
Resources:
|
Categories
All
|