Cancer Education and Research Institute (CERI)'s Cancer Education Programs
Cancer Education and Research Institute®(CERI)'s, formerly Cancer Research Simplified, mission is simple: Cancer Education for All™! Our Team of cancer experts provides you with the most reliable, most up-to-date, and simplified cancer information available. Our educational programs quickly became trusted and highly sought after worldwide.
CERI strongly stands behind the importance of cancer patient empowerment and is a worldwide leading source for simplified, multi-language cancer education. Knowledge is power and empowerment is the key for greater treatment success, early diagnosis, as well as cancer prevention.
For any questions or requests, please contact us.
CERI strongly stands behind the importance of cancer patient empowerment and is a worldwide leading source for simplified, multi-language cancer education. Knowledge is power and empowerment is the key for greater treatment success, early diagnosis, as well as cancer prevention.
For any questions or requests, please contact us.
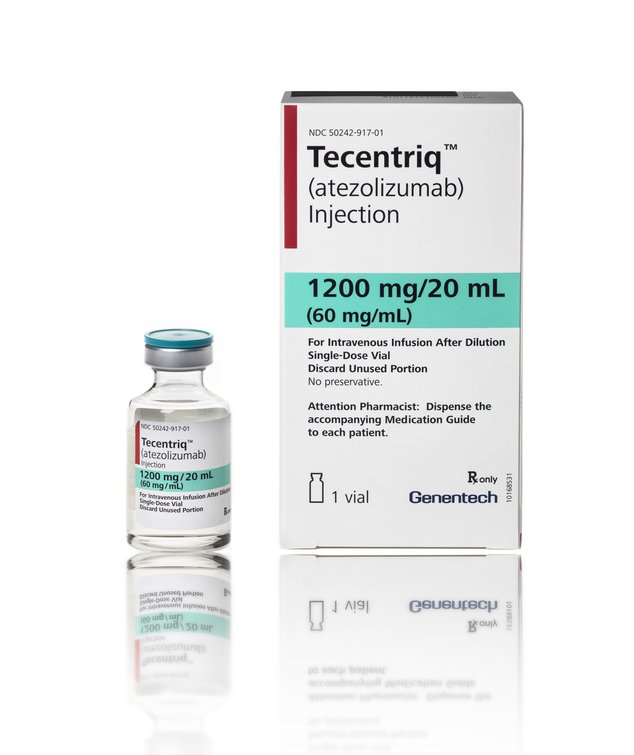
FDA approves immunotherapy treatment for bladder cancer: TECENTRIQ® (atezolizumab)
By Ayguen Sahin, M.Sc., Ph.D. |Cancer Education and Research Institute (CERI)
The U.S. Food and Drug Administration (FDA) approved on May 18, 2016 TECENTRIQ® (atezolizumab) to treat urothelial carcinoma (UCC) [a.k.a Transitional cell carcinoma (TCC)], the most common type of bladder cancer. This is the first product in its class (PD-1/PD-L1 inhibitors) approved to treat this type of cancer.
What is Tecentriq?
|
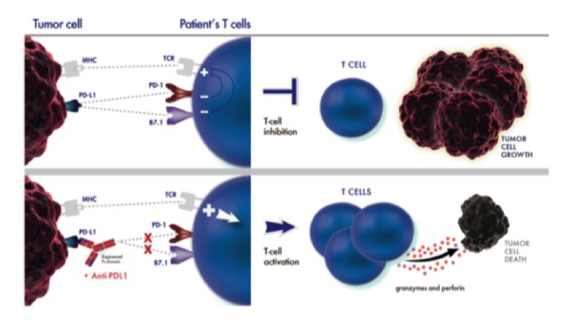
TECENTRIQ® is the first PD-L1 inhibitor approved by the FDA. It is a monoclonal antibody (immunotherapeutic drug) that is designed to target the PD-L1 pathwayby binding to a protein called PD-L1, which is expressed on tumor cells and tumor-infiltrating immune cells. TECENTRIQ blocks the interaction of PD-L1with both PD-1 and B7.1 receptors (proteins that are naturally binding specifically to PD-L1 and activate tumor cells or tumor-infiltrating cells). By inhibiting PD-L1, TECENTRIQ may enable the activation of T cells to attack and kill cancer cells.
Targeting PD-L1 will block the interaction of PD-1 with PD-L1 (PD-1/PD-L1 pathway), which we also call checkpoint inhibitor pathway. By nature, checkpoint inhibitors are way to help the immune system not to attack the body's own cells. By blocking these interactions, Tecentriq may help activating body’s immune system to attack cancer cells. Tecentriq is the first FDA-approved PD-L1 inhibitor as well as the latest in the broader class of PD-1/PD-L1 targeted biologics approved by the FDA in the last two years. Of note: TECENTRIQ is approved in the European Union, United States and more than 50 countries for people with previously treatedmetastatic non-small cell lung cancer (NSCLC) and peoplewith locally advanced or metastatic urothelial cancer (mUC). For urothelial cancer eligibility, see the section ‘For whom is Tecentriq?’ below. |
For whom is Tecentriq?
Tecentriq is approved for the treatment of patients with locally advanced or metastatic urothelial cancer (mUC) who are not eligible for cisplatin chemotherapy, or who have had disease progression (disease has worsened) during or following platinum-containing therapy.
What is urothelial carcinoma?
|
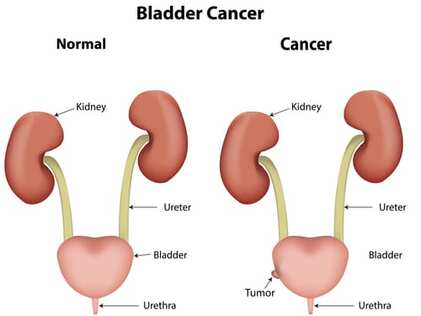
Urothelial carcinoma is the most common type of bladder cancer and occurs in the urinary tract system, involving the bladder, ureter, and urethra. Bladder stores the urine, which is the waste of liquid produced by the kidneys and transferred to the bladder by two tubes called ureter. When the muscles in the bladder contract and you urinate, the liquid (urine) is forced out of the bladder through a tube called ureter (see picture above).
Please note: Besides urothelial carcinoma, there are several other types of bladder cancer such as squamous cell carcinoma, adenocarcinoma, small cell carcinoma, and sarcoma. This FDA approval involves only urothelial carcinoma. To learn more about this carcinoma, watch our ‘What is carcinoma?’ video. 👉 |
|
What are the statistics for bladder cancer?
|
The National Cancer Institute (NCI) estimates 76,960 new cases of bladder cancer and 16,390 deaths from the disease in 2016. In 2019, the NCI estimates 80,470 new cases of bladder cancer and 17,670 deaths from this disease. Between 2009-2015, the survival rate for bladder cancer was 77.1%.
The safety and efficacy of TecentriqThe safety and efficacy of Tecentriq were studied in a single-arm clinical trial, in which everyone enrolled in a trial received the experimental therapy (common in Phase 1 and 2 testing). This study involved 310 patientswith locally advanced or metastatic urothelial carcinoma.
This trial measured the percentage of patients who experienced complete or partial shrinkage of their tumors, which is called objective response rate. The study also looked at the difference in effect based on “positive” versus “negative” expression of the PD-L1 protein (meaning, whether or not the PD-L1 protein exists) on patients’ tumor-infiltrating immune cells. In all patients, 14.8% of participants experienced at least a partial shrinkage of their tumors. This effect lasted from more than 2.1 to more than 13.8 months at the time of the response analysis. In patients who were classified as “positive” for PD-L1 expression (meaning, the PD-L1 exists on patient’s tumor-infiltrating immune cells), 26% of participants experienced a tumor response. The tumor response was only 9.5% in participants who were classified as “negative” for PD-L1 expression (meaning, the PD-L1 does notexist on patient’s tumor-infiltrating immune cells). |
How is PD-L1 detected in positive tumors?
Patients who received Tecentriq had a tumor response throughout the study. However, patients who were “positive” for PD-L1 experienced a greater effect in tumor response. This suggests that the level of PD-L1 expression in tumor-infiltrating immune cells may help identify patients who are more likely to respond to treatment with Tecentriq. Therefore, the FDA also approved the Ventana PD-L1 (SP142) assay to detect PD-L1 protein expression levels on patients’ tumor-infiltrating immune cells and help physicians determine which patients may benefit most from treatment with Tecentriq.
What are the most common side effects of Tecentriq?
The most common side effects of treatment with Tecentriq were fatigue, decreased appetite, nausea, urinary tract infection, fever (pyrexia) and constipation. However, Tecentriq also has the potential to cause infection and ‘immune-mediated side effects’, which are serious side effects as a result of the effect of Tecentriq on the immune system. These severe immune-mediated side effects involve healthy organs, including the lung, colon and endocrine system.
Approval of Tecentriq
The FDA granted the Tecentriq application breakthrough therapy designation, priority review status and accelerated approval for this indication.
Who markets Tecentriq?
Tecentriq is marketed by Genentech based in San Francisco, California. The Ventana PD-L1 (SP142) assay complementary diagnostic for Tecentriq is marketed by Ventana Medical Systems, based in Tucson, Arizona.
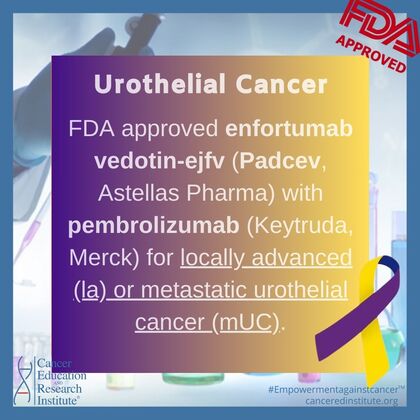
Further FDA Approvals for Bladder Cancer
December 15, 2023:
References:
- https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm501762.htm Accessed on October 23, 2018.
- TECENTRIQ® (atezolizumab), Roche. https://www.roche.com/products/product-details.htm?productId=8c738504-101a-40b9-867b-d907c578df51Accessed on May 21, 2019.
- Bladder cancer statistics, the National Cancer Institute (NCI). https://seer.cancer.gov/statfacts/html/urinb.html Accessed on May 21, 2019.
- Ventana PD-L1 (SP142) Assay. https://www.fda.gov/medical-devices/recently-approved-devices/ventana-pd-l1-sp142-assay-p160002s009 Accessed on May 21, 2019.
- FDA approves enfortumab vedotin-ejfv with pembrolizumab for locally advanced or metastatic urothelial cancer (accessed on December 19, 2023)
#bladdercancer #urothelialcarcinoma #immunotherapy #urotherialcancer #fda #fdaapproval #PDL1 #PDL1inhibitor #tecentriq #atezolizumab #checkpointinhibitors #PD1 #cancer #cancerdrug #cancertreatment #clinicaltrials #bladdercancerawareness #bladdercancerawarenessmonth #cancereducation
👉 Like our Facebook page for daily cancer info: Cancer Education and Research Institute
#canceredinstitute
#canceredinstitute
You might also like:
|
|
|
Disclaimer: We can not assume responsibility of misinterpretation of content.
Please donate to support our cause - help us continue with our trusted educational programs:
Cancer Education and Research Institute, fka Cancer Research Simplified, is a registered 501(c)(3) non-profit organization.
Your donations are tax deductible to the extent permitted by law.
Your donations are tax deductible to the extent permitted by law.