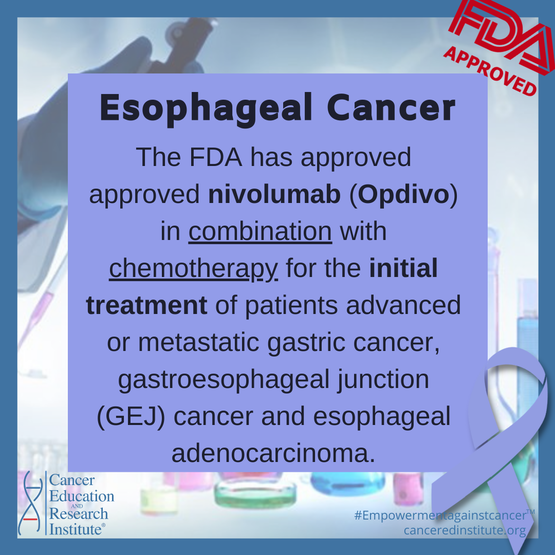
This is the first FDA-approved immunotherapy for the first-line treatment of gastric cancer.
- How does Opdivo (nivolumab) work? Opdivo is a monoclonal antibody that inhibits tumor growth by enhancing T-cell function.
- How many patients were evaluated for this study? 1,581
- Median overall survival (OS) for the pembrolizumab + chemotherapy arm: 13.8 months
- Median overall survival (OS) for the chemotherapy arm: 11.6 months
- What were the most common adverse reactions? The most common side effects of Opdivo in combination with chemotherapy include:
- peripheral neuropathy (damage to the nerves outside of the brain and spinal cord),
- nausea,
- fatigue,
- diarrhea,
- vomiting,
- decreased appetite,
- abdominal pain,
- constipation,
- musculoskeletal pain.
- Opdivo can cause serious conditions known as immune-mediated side effects, including:
- inflammation of healthy organs such as the lungs (pneumonitis), colon (colitis), liver (hepatitis), endocrine glands (endocrinopathies) and kidneys (nephritis).
- Patients should tell their healthcare providers if they have immune system problems, lung or breathing problems, liver problems, have had an organ transplant, or are pregnant or plan to become pregnant before starting treatment.
Reference:
- https://www.fda.gov/news-events/press-announcements/fda-approves-first-immunotherapy-initial-treatment-gastric-cancer?s=03 (accessed April 16, 2021).