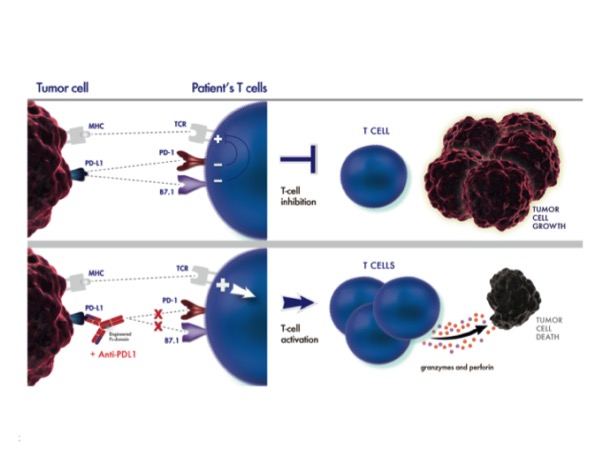
Tecentriq targets the PD-L1 pathway. Targeting PD-L1 will block the interaction of PD-1 with PD-L1 (PD-1/PD-L1 pathway), which we also call checkpoint inhibitor pathway. By nature, checkpoint inhibitors are way to help the immune system not to attack the body's own cells. By blocking these interactions, Tecentriq may help activating body’s immune system to attack cancer cells. Tecentriq is the first FDA-approved PD-L1 inhibitor as well as the latest in the broader class of PD-1/PD-L1 targeted biologics approved by the FDA in the last two years.
What is urothlial carcinoma? Urothelial carcinoma is the most common type of bladder cancer and occurs in the urinary tract system, involving the bladder and related organs. The National Cancer Institute (NCI) estimates 76,960 new cases of bladder cancer and 16,390 deaths from the disease in 2016.
How to detect PD-L1 positive tumors: While patients who received Tecentriq experienced a tumor response across the study, the greater effect in those who were classified as “positive” for PD-L1 expression suggests that the level of PD-L1 expression in tumor-infiltrating immune cells may help identify patients who are more likely to respond to treatment with Tecentriq. Therefore, the FDA also approved the Ventana PD-L1 (SP142) assay to detect PD-L1 protein expression levels on patients’ tumor-infiltrating immune cells and help physicians determine which patients may benefit most from treatment with Tecentriq.
Most common side effects of Tecentriq: The most common side effects of treatment with Tecentriq were fatigue, decreased appetite, nausea, urinary tract infection, fever (pyrexia) and constipation. Tecentriq also has the potential to cause infection and serious side effects that result from the immune system effect of Tecentriq (known as “immune-mediated side effects”). These severe immune-mediated side effects involve healthy organs, including the lung, colon and endocrine system.
Approval of Tecentriq: The FDA granted the Tecentriq application breakthrough therapy designation, priority review status and accelerated approval for this indication.
Who markets Tecentriq? Tecentriq is marketed by Genentech based in San Francisco, California. The Ventana PD-L1 (SP142) assay complementary diagnostic for Tecentriq is marketed by Ventana Medical Systems, based in Tucson, Arizona.
Source: The U.S. Food and Drug Administration (FDA)