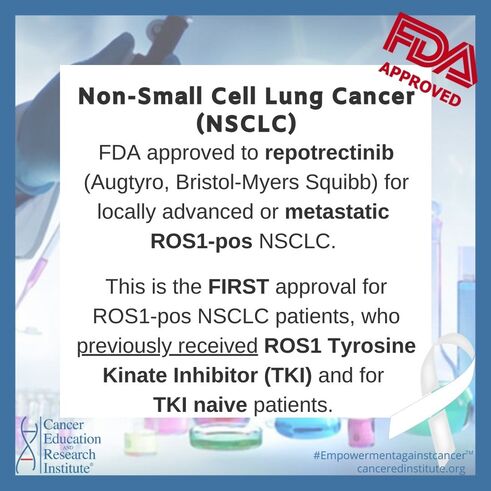
Approval was based on the clinical trial called TRIDENT-1 (NCT03093116), which is a global, multicenter, single-arm, open-label, multi-cohort clinical trial. This trial included patients with ROS1-positive locally advanced or metastatic NSCLC.
- 71 ROS1 TKI-naïve patients who received up to 1 prior line of platinum-based chemotherapy and/or immunotherapy and,
- 56 patients who received 1 prior ROS1 TKI with no prior platinum-based chemotherapy or immunotherapy.
Confirmed overall response rate (ORR):
- In the ROS1 TKI naïve group: 79% (95% CI: 68, 88)
- In the prior treatment with a ROS1 inhibitor group: 38% (95% CI: 25, 52)
Median duration of response (DOR):
- In the ROS1 TKI naïve group: 34.1 months (95% CI: 25.6, not evaluable)
- In the prior treatment with a ROS1 inhibitor group: 14.8 months (95% CI: 7.6, not evaluable)
Responses were observed in intracranial lesions in patients with measurable Central Nervous System (CNS) metastases, and in patients with resistance mutations following TKI therapy.
Side Effects:
The most common (more than 20%) adverse reactions were:
- dizziness
- dysgeusia
- peripheral neuropathy
- constipation
- dyspnea
- ataxia
- fatigue
- cognitive disorders
- muscular weakness
Recommended Dose:
The recommended repotrectinib dose is 160 mg orally once daily with or without food for 14 days, then increased to 160 mg twice daily, until disease progression or unacceptable toxicity.
#CERICancerEducation
#FDAapprovals
If you need help for your cancer treatment and care, apply to our CERI Personalized Patient Program™:
canceredinstitute.org/patient-program
Resources:
FDA approves repotrectinib for ROS1-positive non-small cell lung cancer
#NSCLC #nonsmallcelllungcancer #ROS1pos #ROS1posNSCLC #repotrectinib #Augtyro #BristolMyersSquibb #TKInaive #metastaticcancer #metastasis #metastaticNSCLC #cancertreatment #fdaapproves #fdaapproved #cancertherapy