Cancer Education and Research Institute (CERI)'s Cancer Education Programs
Our Team of cancer experts at Cancer Education and Research Institute® (CERI)'s, formerly Cancer Research Simplified, provides you with the most reliable, most up-to-date, and simplified cancer information available. Our educational programs quickly became trusted and highly sought after worldwide.
CERI strongly stands behind the importance of cancer patient empowerment and is a worldwide leading source for simplified, multi-language cancer education. Knowledge is power and empowerment is the key for greater treatment success, early diagnosis, as well as cancer prevention.
For any questions or requests, please submit your inquiry at our CERI Personalized Patient Program™ page.
CERI strongly stands behind the importance of cancer patient empowerment and is a worldwide leading source for simplified, multi-language cancer education. Knowledge is power and empowerment is the key for greater treatment success, early diagnosis, as well as cancer prevention.
For any questions or requests, please submit your inquiry at our CERI Personalized Patient Program™ page.
FDA Approved Genetic Screening Test for Cervical Cancer
Elana Meer | Cancer Education and Research Institute (CERI)
Aygün Sahin, MSc, PhD | CEO, President, and Cancer Lead, Cancer Education and Research Institute (CERI)
Aygün Sahin, MSc, PhD | CEO, President, and Cancer Lead, Cancer Education and Research Institute (CERI)
What is Cervical Cancer?
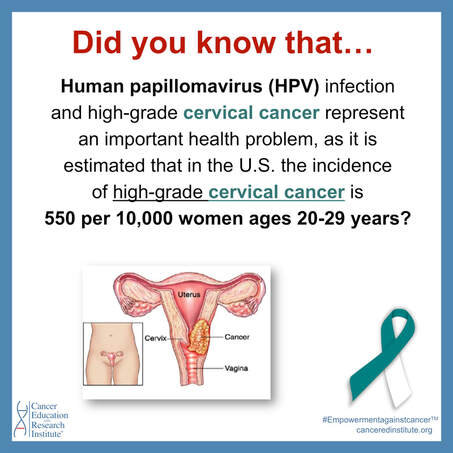
Cervical Cancer is a growth of abnormal cells that forms in the tissues of the cervix. Most cervical cancers are derived from a human papillomavirus (HPV) infection. HPV is an extremely common sexually transmitted infection. There are about 150 human papilloma viruses, most of which are causing only minor health problems; however, there are 15 high-risk types, that are associated with cervical cancer. Certain strains of the virus, such as HPV16 and HPV18, are responsible for 70% of cervical cancer.
Cervical Cancer Facts
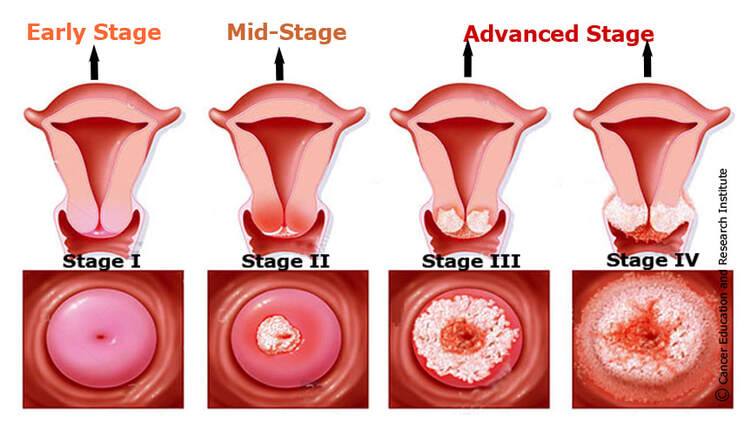
Cervical Cancer Stages
The Pap Test
The Pap test is a minimally invasive procedure which involves scraping cells from the lining of the cervix with a medical instrument and then looking at these cell samples under microscopes to examine changes in cells that might evolve into cervical cancer. Cervical cancer grows slowly, and because it shows minimal symptoms, it is difficult to detect without consistent and effective testing. In the past, the main screening option to detect cervical cancer was the Pap smear (“Pap test”). The Pap test method of screening and detecting cervical cancers has resulted in a more than 50% decrease in the number of cervical cancer cases in the past 30 years.
The HPV Test
The HPV test, a new option for cervical cancer screening developed by Roche Molecular Systems, has recently been approved by the Food and Drug Administration. This test involves invasively taking a sample of cervical tissue enabling the DNA from 24 high-risk HPV types to be detected In particular, the test was able to identify in a safe and effective way, the particular strains most likely to lead to cervical cancer: HPV16 and HPV18. Although as of now HPV tests cost about twice as much as Pap tests, HPV testing has the capacity to determine whether or not a woman should undergo additional diagnostic testing for cervical cancer, and to give information regarding a patient’s risk for developing the cervical cancer in the future. For instance, women who have HPV16 or 18, as well as some women who are infected with one of the other high-risk HPV types, should obtain a further colposcopy test.
Medical Guidelines for Cervical Testing
Medical guidelines for cervical testing should not be drastically changed by FDA approval of this new testing method. We now just have another option for cervical cancer screening. Used in tandem with patient screening history and risk factors, both the Pap and HPV test techniques may be incorporated to improve the efficacy and accuracy of cervical cancer detection. As HPV testing is more invasive and can potentially cause the cervix to be less able to handle pregnancy later in life, suggested testing combinations include a Pap test every 3 years for women between the ages of 21 and 29, and a Pap and HPV test every 5 years for women over 30. The FDA has still not specified the long-term side effects of HPV testing or if there is a limit to the number of HPV tests a person should have; however, it has proceeded through thorough testing, in a trial with over 47000 women, to prove its efficacy and safety. This new HPV test is a promising development in the expansion of cervical cancer screening, and may allow women to make more informed decisions about their health.
Cervical Cancer Prevention, HPV vaccines
Supplementary CERI Information
For more information on the different stages and facts of ovarian cancer, please visit our ovarian cancer series from the intro, episode 18, to subsequent episodes 19-23.
#Empowermentagainstcancer™ #CERICares #CERIinforms #CancerSimplified
#cervicalcancer #womenshealth #paptest #hpvtest #hpv #hpvvaccines #gardasil #cervarix #gynecologiccancers #cancereducation #cancerresearch #cancertreatment #cancerawareness #fdaapprovals
January is Cervical Cancer Awareness Month
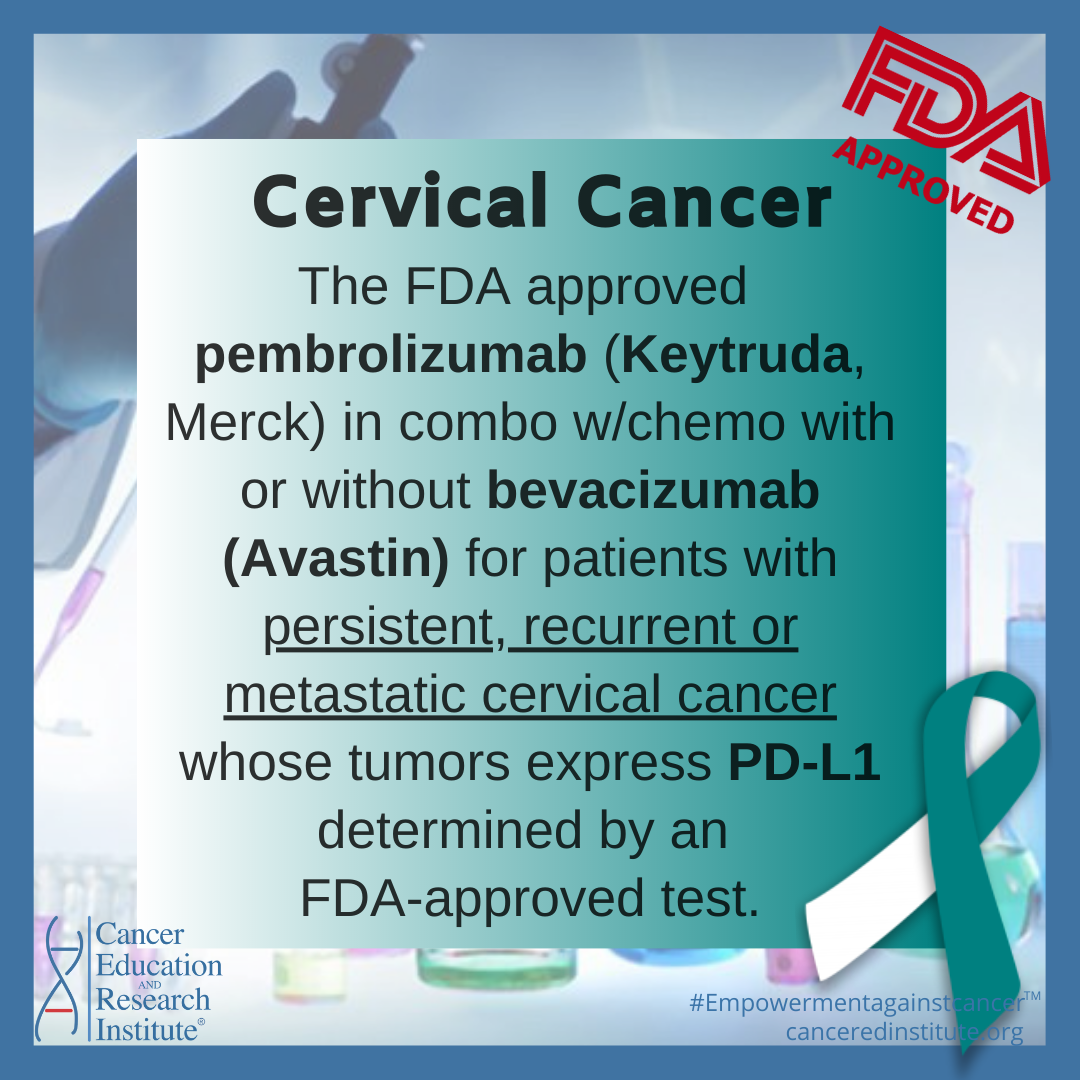
FDA Approvals:
References:
- Cervical Cancer. National Cancer Institute. cancer.gov, n.d. Web. 2 May 2014. http://www.cancer.gov/cancertopics/types/cervical
- HPV and Cancer - National Cancer Institute. HPV. National Cancer Institute, n.d. Web. 3 May 2014. http://www.cancer.gov/cancertopics/factsheet/Risk/HPV
- Press, Matthew. FDA approves genetic screening test for cervical cancer. USA Today. Gannett, 25 Apr. 2014. Web. 2 May 2014. http://www.usatoday.com/story/news/nation/2014/04/25/dna-test-cervical-cancer-pap-smear/8144557
- U.S. Food and Drug Administration. FDA approves first human papillomavirus test for primary cervical cancer screening. N.p., n.d. Web. 3 May 2014. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm394773.htm
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761208s000lbl.pdf (accessed on September 21, 2021)
GET YOUR FREE ARTICLE: Make sure to get your free article as our gift to you! Go to: canceredinstitute.org/ceri-simplified-cancer-research-articles
CORONAVIRUS PAGE: For our educational and up-to-date informational page on Coronavirus, go to: canceredinstitute.org/coronavirus
FOLLOW US for DAILY information and updates:
CORONAVIRUS PAGE: For our educational and up-to-date informational page on Coronavirus, go to: canceredinstitute.org/coronavirus
FOLLOW US for DAILY information and updates:
- INSTAGRAM: instagram.com/canceredinstitute
- FACEBOOK: facebook.com/canceredinstitute
- Twitter: twitter.com/canceredinst
- LinkedIn: linkedin.com/company/canceredinstitute
You might also like:
|
|
|
|
|
|
Disclaimer: We can not assume responsibility of misinterpretation of content.