Cancer Education and Research Institute (CERI)'s Cancer Education Programs
Cancer Education and Research Institute® (CERI)'s, formerly Cancer Research Simplified, mission is simple: Cancer Education for All™! Our Team of cancer experts provides you with the most reliable, most up-to-date, and simplified cancer information available. Our educational programs quickly became trusted and highly sought after worldwide.
CERI strongly stands behind the importance of cancer patient empowerment and is a worldwide leading source for simplified, multi-language cancer education. Knowledge is power and empowerment is the key for greater treatment success, early diagnosis, as well as cancer prevention.
For any questions or requests, please contact us.
CERI strongly stands behind the importance of cancer patient empowerment and is a worldwide leading source for simplified, multi-language cancer education. Knowledge is power and empowerment is the key for greater treatment success, early diagnosis, as well as cancer prevention.
For any questions or requests, please contact us.
Prostate Cancer
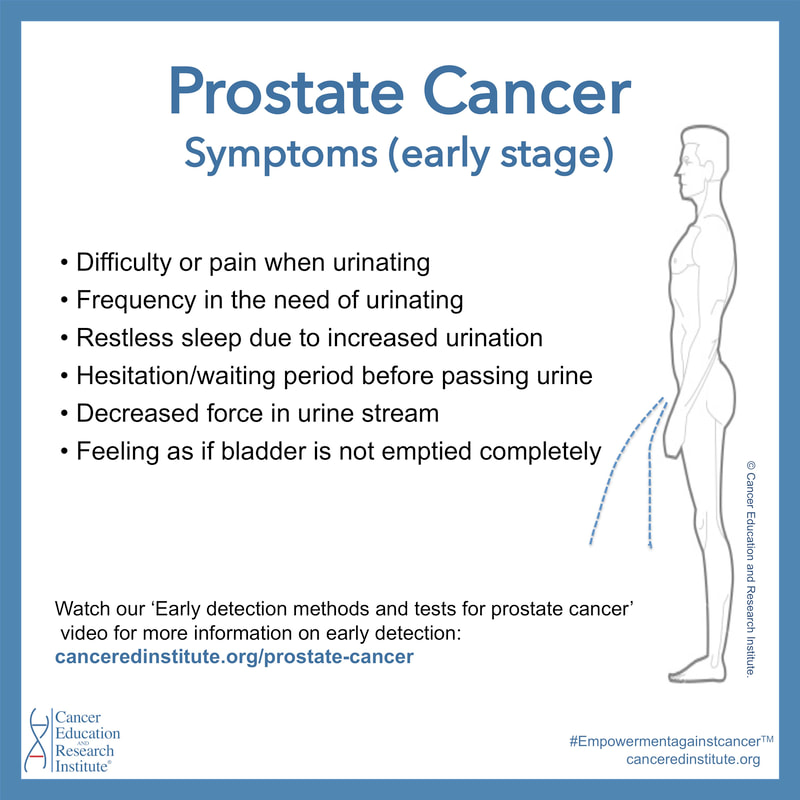
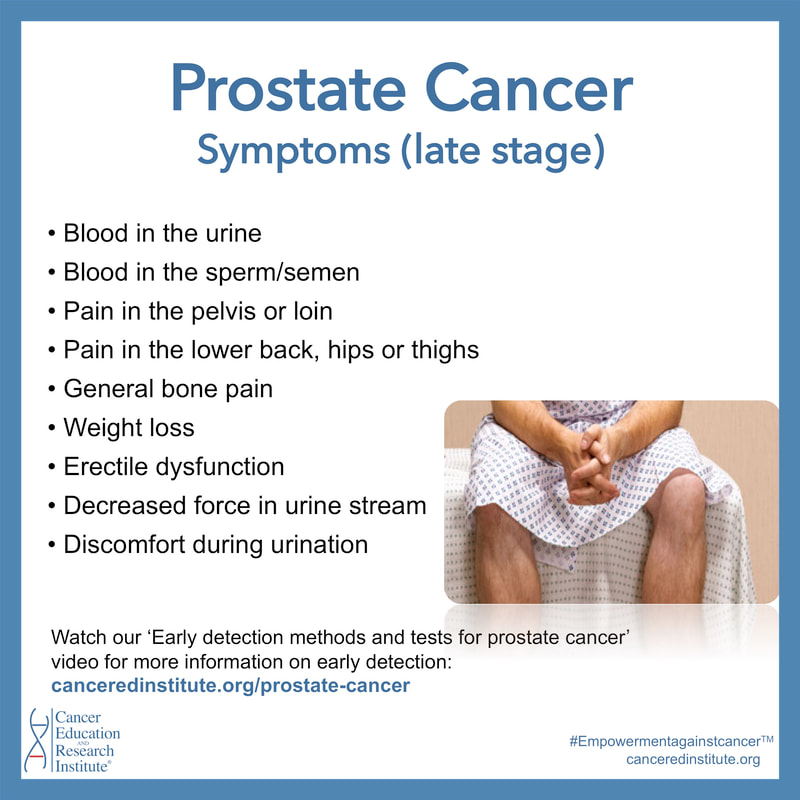
'Possible' symptoms for prostate cancer:
Keep in mind that some (early stage) symptoms related to prostate cancer and Benign Prostatic Hyperplasia (BPH, or enlarged prostate) are often similar. These symptoms are displayed in our educational picture.
Watch our “Early detection methods and tests for prostate cancer” video below to learn more about early detection.
If you have any symptoms that worry you, make an appointment with your doctor. You should discuss the prostate screening and the methods with your doctor, and decide ‘together’ what is best for you.
Keep in mind that some (early stage) symptoms related to prostate cancer and Benign Prostatic Hyperplasia (BPH, or enlarged prostate) are often similar. These symptoms are displayed in our educational picture.
Watch our “Early detection methods and tests for prostate cancer” video below to learn more about early detection.
If you have any symptoms that worry you, make an appointment with your doctor. You should discuss the prostate screening and the methods with your doctor, and decide ‘together’ what is best for you.
|
|
|
FDA Approvals
3/23/2022: FDA approved to PLUVICTO™ (lutetium Lu 177 vipivotide tetraxetan, Novartis) to treat prostate-specific membrane antigen-positive metastatic castration-resistant prostate cancer following other therapies.
PLUVICTO is a radioligand therapeutic agent indicated for the treatment of adult patients with prostate-specific membrane antigen (PSMA)-positive metastatic castration-resistant prostate cancer (mCRPC) who have been treated with androgen receptor (AR) pathway inhibition and taxane-based chemotherapy.
Injection: 1,000MBq/mL (27mCi/mL) in a single-dose vial.
Recommended Dosage: Administer 7.4GBq (200mCi) every 6 weeks for up to 6 doses.
Learn more in the FDA label document here.
PLUVICTO is a radioligand therapeutic agent indicated for the treatment of adult patients with prostate-specific membrane antigen (PSMA)-positive metastatic castration-resistant prostate cancer (mCRPC) who have been treated with androgen receptor (AR) pathway inhibition and taxane-based chemotherapy.
Injection: 1,000MBq/mL (27mCi/mL) in a single-dose vial.
Recommended Dosage: Administer 7.4GBq (200mCi) every 6 weeks for up to 6 doses.
Learn more in the FDA label document here.
In our further video, we explain several FDA approved a new radioactive diagnostic imaging agent, Axumin, which was approved to detect recurrent prostate cancer. You can also read our article on our website.
#ProstateCancer #Cancer #earlydetectionofcancer #earlydetection #cancerawareness #prostatecancersymptoms #BenignProstaticHyperplasia #BPH #enlargedprostate #Prostatakrebs #Prostatkanseri #cancereducation #cancersimplified
You might also like:
|
|
|
Disclaimer: We can not assume responsibility of misinterpretation of content.