Cancer Education and Research Institute (CERI)'s Cancer Education Programs
Our Team of cancer experts at Cancer Education and Research Institute® (CERI)'s, formerly Cancer Research Simplified, provides you with the most reliable, most up-to-date, and simplified cancer information available. Our educational programs quickly became trusted and highly sought after worldwide.
CERI strongly stands behind the importance of cancer patient empowerment and is a worldwide leading source for simplified, multi-language cancer education. Knowledge is power and empowerment is the key for greater treatment success, early diagnosis, as well as cancer prevention.
For any questions or requests, please submit your inquiry at our CERI Personalized Patient Program™ page.
CERI strongly stands behind the importance of cancer patient empowerment and is a worldwide leading source for simplified, multi-language cancer education. Knowledge is power and empowerment is the key for greater treatment success, early diagnosis, as well as cancer prevention.
For any questions or requests, please submit your inquiry at our CERI Personalized Patient Program™ page.
Breast Cancer Treatments
Aygün Sahin, MSc, PhD | CEO and Cancer Lead, Cancer Education and Research Institute (CERI)
This page will be updated regularly!
FDA approved treatments for Breast Cancer
FDA approvals in 2021
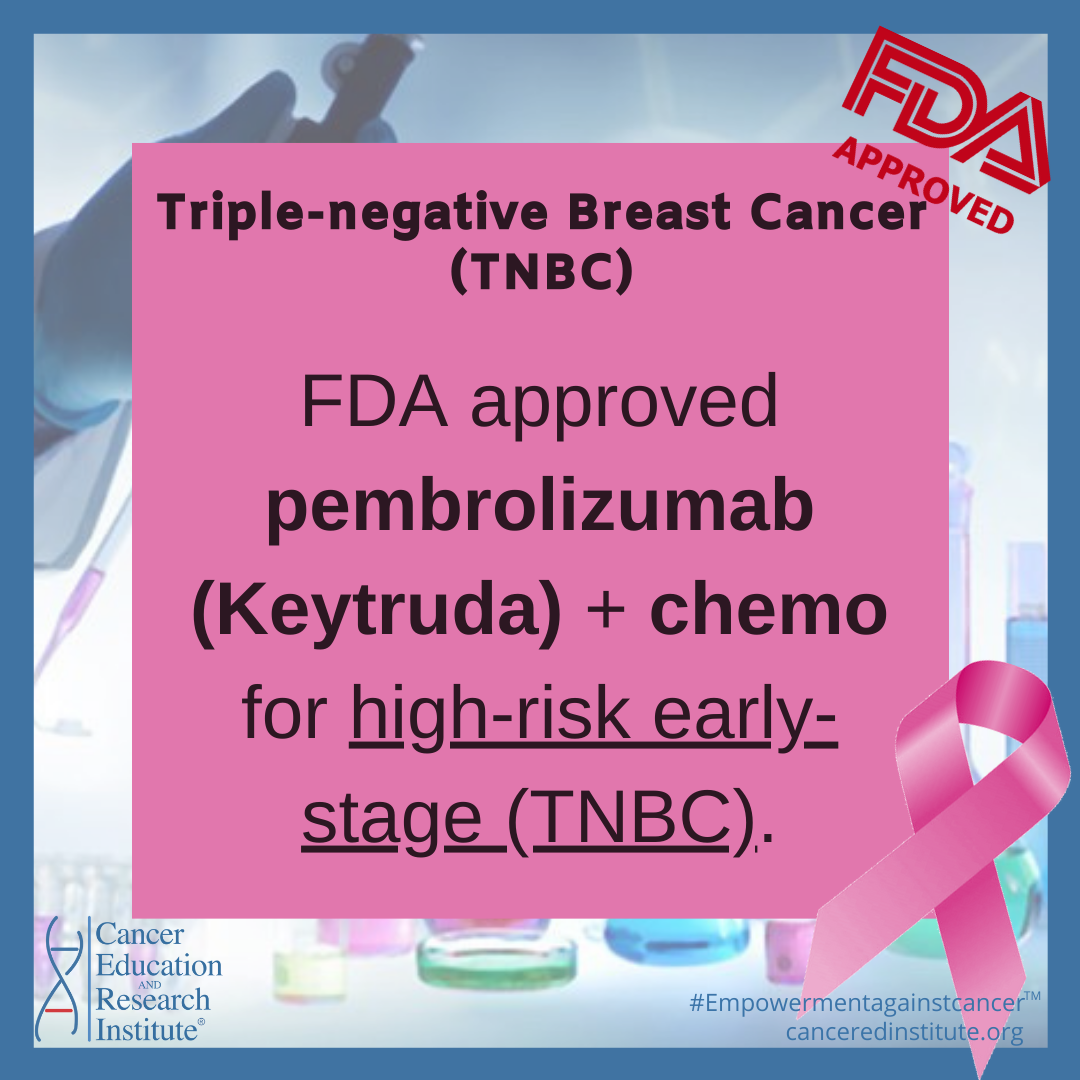
- July 26, 2021: The FDA approved pembrolizumab for high-risk early-stage triple-negative breast cancer
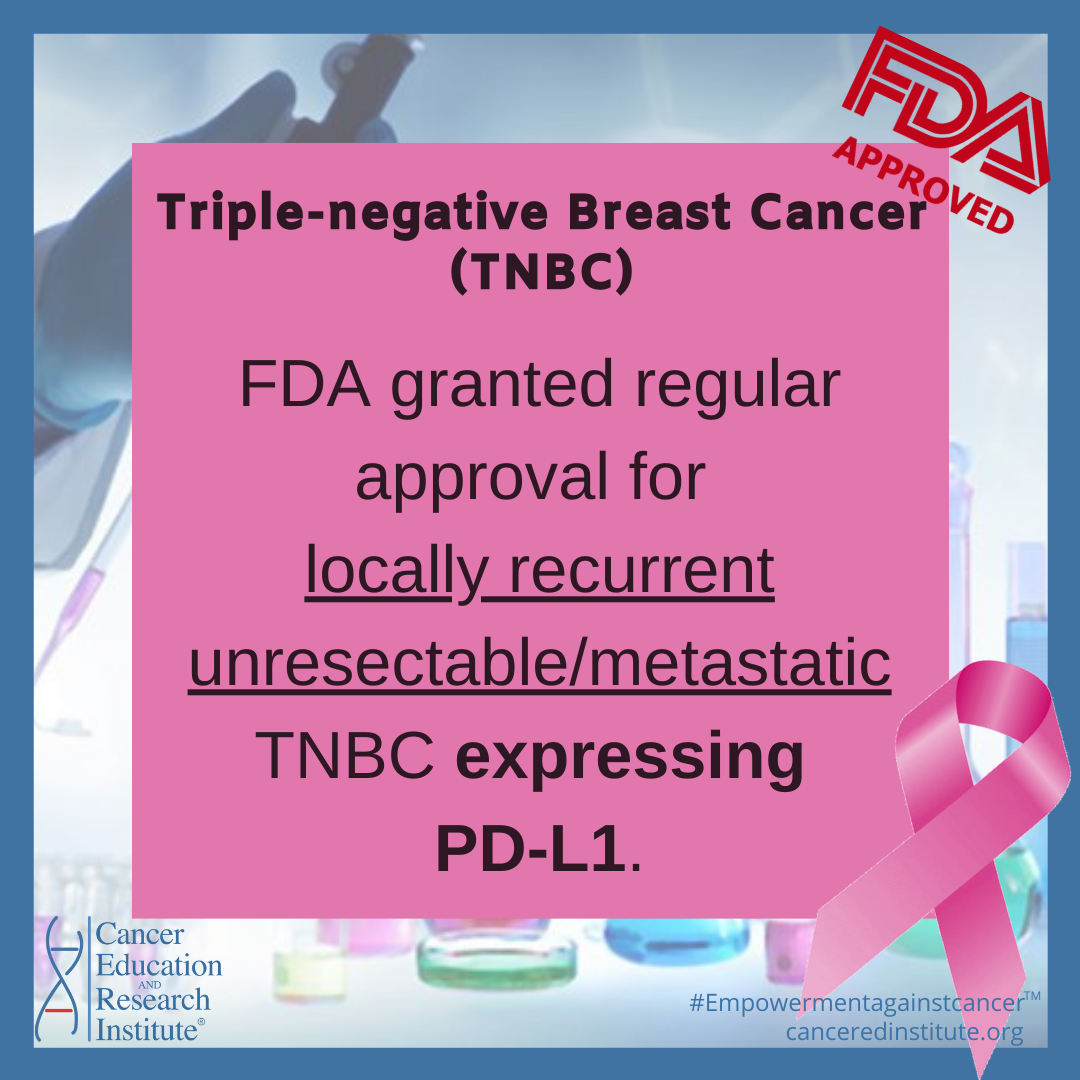
The FDA also granted regular approval to pembrolizumab in combination with chemotherapy for patients with locally recurrent unresectable or metastatic TNBC whose tumors express PD-L1 as determined by an FDA approved test.
FDA granted accelerated approval to pembrolizumab for this indication in November 2020.
The results of each of the clinical trials are as follow:
Pathological complete response (pCR) rate:
Patients who received pembrolizumab in combination with chemotherapy: 63% (95% CI: 59.5, 66.4)
Patients who received chemotherapy alone: 56% (95% CI: 50.6, 60.6)
Event free survival (EFS):
Patients who received pembrolizumab in combination with chemotherapy: 123% (16% CI: 59.5, 66.4)
Patients who received chemotherapy alone: 93% (24% CI: 50.6, 60.6)
HR 0.63; 95% CI: 0.48, 0.82; p=0.00031
FDA approvals in 2020
- December 16, 2020: FDA approved MARGENZA for treatment of metastatic HER2-positive breast cancer
Learn more here.
- May 20, 2020: FDA approved diagnostic imaging agent, Cerianna, for estrogen receptor (ER)-positive patients with breast cancer
Learn more here.
#breastcancer #breastcancertreatment #estrogenreceptorpositivebreastcancer #ERpostitivebreastcancer #BC #BCSM #fda #fdaapproved #fdaapproval #today #keytruda
Resources:
- https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-high-risk-early-stage-triple-negative-breast-cancer?utm_medium=email&utm_source=govdelivery (accessed on July 27, 2021)
- https://www.fda.gov/drugs/drug-approvals-and-databases/drug-trials-snapshot-margenza (accessed on July 27, 2021)
- https://www.fda.gov/drugs/drug-approvals-and-databases/drug-trial-snapshot-cerianna (accessed on July 27, 2021)
Click here to learn everything you need to know about breast cancer and how to do breast self-examination (BSE) on our Youtube channel.
You might also like:
|
|
|
|
|
|
Disclaimer: We can not assume responsibility of misinterpretation of content.