The Journal of Simplified Cancer Research (JSCR) - New Article Release
Acute Myelocytic Leukemia (AML) Therapies
By Ayguen Sahin, MSc, PhD | Cancer Education and Research Institute (CERI)
December 13, 2022
FDA Approvals for AML
December 1, 2022:
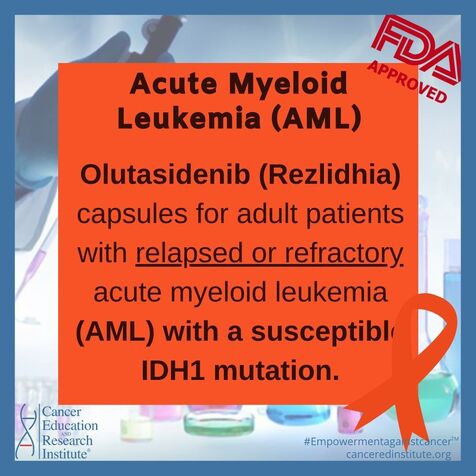
FDA approves olutasidenib for relapsed or refractory acute myeloid leukemia with a susceptible IDH1 mutation
FDA approves olutasidenib for relapsed or refractory acute myeloid leukemia with a susceptible IDH1 mutation
The Food and Drug Administration (FDA) approved olutasidenib (Rezlidhia) capsules for adult patients with relapsed or refractory acute myeloid leukemia (AML) with a susceptible IDH1 mutation as detected by an FDA-approved test.The FDA also approved the Abbott RealTime IDH1 Assay to select patients for olutasidenib.
Read more here.
Read more here.
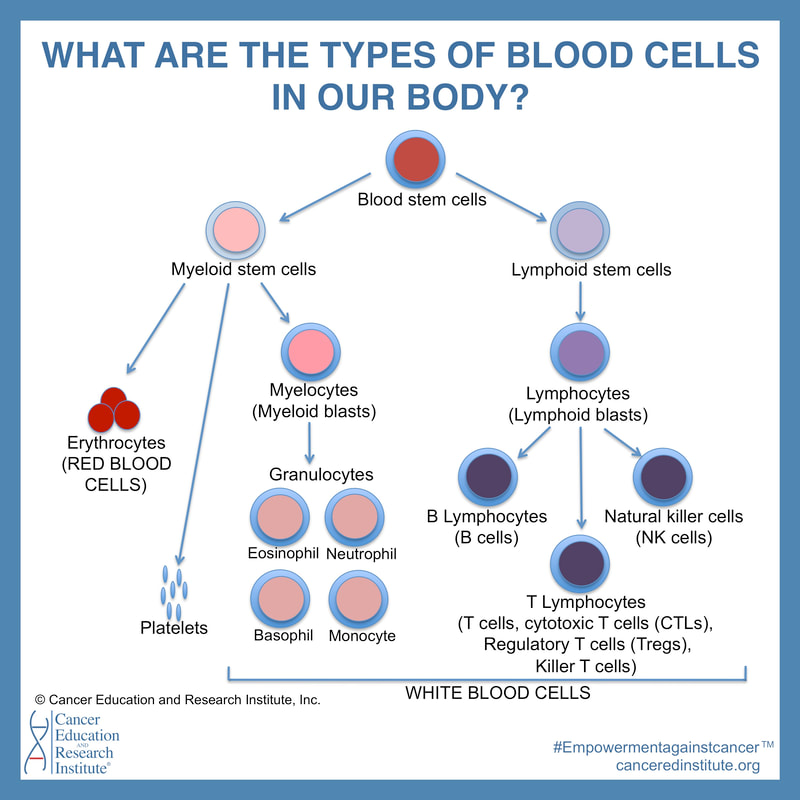
What are the types of blood cells?
The Journal of Simplified Cancer Research (JSCR) is the official journal of Cancer Research Simplified. For only $4.99 per monthly issue, [or a discounted $49.99 per year], you can gain digital access to straightforward information and articles on cancer news, diagnosis, prevention, treatment, and clinical trials. Fill out the form, make your payment, and you'll receive your copy right in your inbox! To get your copy now on The Journal of Simplified Cancer Research (JSCR) page.