The Journal of Simplified Cancer Research (JSCR) - New Article Release
VEGF-Targeting Ramucirumab Receives Approval as Drug for Metastatic Colorectal Cancer
By Jenna Newman | CERI Research Team
More details of this article can be found in our JSCR - July 2015 Issue
More details of this article can be found in our JSCR - July 2015 Issue

CYRAMZA® (ramucirumab)
Eli Lilly and Company’s CYRAMZA® (ramucirumab), a drug designed to treat metastatic colorectal cancer, was approved by the Food and Drug Administration (FDA) of the United States on April 24, 2015 [1]. Ramucirumab is administered to patients who have previously taken bevacizumab, oxaliplatin, and fluoropyrimidine-based treatment(s) and have not demonstrated halted progression of metastasis [1]. Metastasis is the spread of cancerous cells from the original local site, at which a tumor originates to distant sites in the body [2].
Eli Lilly and Company’s CYRAMZA® (ramucirumab), a drug designed to treat metastatic colorectal cancer, was approved by the Food and Drug Administration (FDA) of the United States on April 24, 2015 [1]. Ramucirumab is administered to patients who have previously taken bevacizumab, oxaliplatin, and fluoropyrimidine-based treatment(s) and have not demonstrated halted progression of metastasis [1]. Metastasis is the spread of cancerous cells from the original local site, at which a tumor originates to distant sites in the body [2].
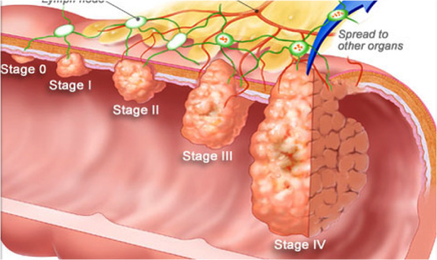 Figure 1. The progression of colorectal cancer
As colorectal cancer advances to the metastatic stage, tumor cells are able to escape from the colon and colonize other areas of the body, often leaving the colon via blood vessels. Ramucirumab is a novel drug that inhibits blood vessel formation, depriving tumors of the nutrients they require for growth.
Figure 1. The progression of colorectal cancer
As colorectal cancer advances to the metastatic stage, tumor cells are able to escape from the colon and colonize other areas of the body, often leaving the colon via blood vessels. Ramucirumab is a novel drug that inhibits blood vessel formation, depriving tumors of the nutrients they require for growth.
What is Ramucirumab?
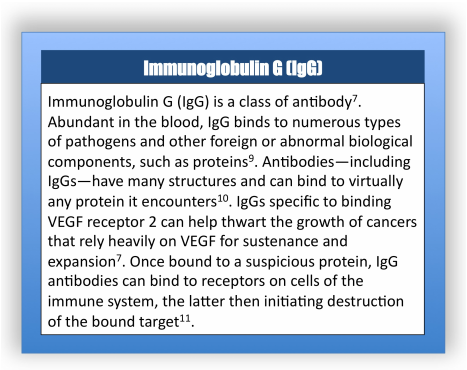
Ramucirumab is a member of an emerging class of cancer drugs in which antibodies—proteins of the immune system that bind with high specificity to particular molecules [3]—are employed to engulf proteins associated with cancer progression, signaling their destruction [4]. Research has shown that metastatic colorectal cancers often overexpress, in comparison to healthy cells, a protein called the vascular endothelial growth factor (VEGF)5. VEGF stimulates growth of blood vessels; tumors overexpressing VEGF, as a result, are nourished by blood and can grow, and ultimately metastasize [5]. Ramucirumab is an antibody of a structural class labeled IgG (Immunoglobulin G) that specifically binds to a portion of a VEGF receptor (a receiving molecule located on the surface of the cell) [6] called VEGF receptor 2 [7]. The VEGF receptor 2, which normally initiates the cascade of actions leading to the sprouting—called angiogenesis—of new blood vessels, cannot operate when bound by the anti-VEGF IgG antibody (known as ramucirumab) [8]. Ramucirumab is designed to reduce angiogenesis in metastatic colorectal cancer patients, thereby shrinking the size of tumors and slowing metastasis [7]. Ultimately, existing tumors are deprived of nutrients from the blood, and should be less likely to grow and metastasize upon treatment with ramucirumab [7].
Ramucirumab is a member of an emerging class of cancer drugs in which antibodies—proteins of the immune system that bind with high specificity to particular molecules [3]—are employed to engulf proteins associated with cancer progression, signaling their destruction [4]. Research has shown that metastatic colorectal cancers often overexpress, in comparison to healthy cells, a protein called the vascular endothelial growth factor (VEGF)5. VEGF stimulates growth of blood vessels; tumors overexpressing VEGF, as a result, are nourished by blood and can grow, and ultimately metastasize [5]. Ramucirumab is an antibody of a structural class labeled IgG (Immunoglobulin G) that specifically binds to a portion of a VEGF receptor (a receiving molecule located on the surface of the cell) [6] called VEGF receptor 2 [7]. The VEGF receptor 2, which normally initiates the cascade of actions leading to the sprouting—called angiogenesis—of new blood vessels, cannot operate when bound by the anti-VEGF IgG antibody (known as ramucirumab) [8]. Ramucirumab is designed to reduce angiogenesis in metastatic colorectal cancer patients, thereby shrinking the size of tumors and slowing metastasis [7]. Ultimately, existing tumors are deprived of nutrients from the blood, and should be less likely to grow and metastasize upon treatment with ramucirumab [7].
 Figure 2. VEGF stimulates angiogenesis, creation of new blood vessels
Figure 2. VEGF stimulates angiogenesis, creation of new blood vessels
How effective is ramucirumab?
Clinical trials have demonstrated that ramucirumab likely prolongs survival—when compared to other chemotherapies and treatments—of metastatic colorectal cancer patients [7]. Patients with advanced diseases receiving ramucirumab survived a median of 13.3 months after treatment, while those receiving other standard chemotherapies for metastatic colorectal cancer lived for a median of 11.7 months after treatment [7]. This is considered a significant improvement [7], and has the potential to prolong the lives of patients with metastatic colorectal cancer. In the REGARD clinical trial, in which ramucirumab was tested as a drug to treat gastric cancers, similar gains in survival were observed (a median of 5.2 months for patients taking ramucirumab versus a median of 3.8 months for patients administered other chemotherapies) [12]
Clinical trials have demonstrated that ramucirumab likely prolongs survival—when compared to other chemotherapies and treatments—of metastatic colorectal cancer patients [7]. Patients with advanced diseases receiving ramucirumab survived a median of 13.3 months after treatment, while those receiving other standard chemotherapies for metastatic colorectal cancer lived for a median of 11.7 months after treatment [7]. This is considered a significant improvement [7], and has the potential to prolong the lives of patients with metastatic colorectal cancer. In the REGARD clinical trial, in which ramucirumab was tested as a drug to treat gastric cancers, similar gains in survival were observed (a median of 5.2 months for patients taking ramucirumab versus a median of 3.8 months for patients administered other chemotherapies) [12]
What are some side effects of ramucirumab?
Patients who participated in clinical trials reported side effects including hypertension, diarrhea, fatigue, and neutropenia after being administered ramucirumab, with 11%, 11%, 12% and 3% of patients having these symptoms, respectively [7].
Patients who participated in clinical trials reported side effects including hypertension, diarrhea, fatigue, and neutropenia after being administered ramucirumab, with 11%, 11%, 12% and 3% of patients having these symptoms, respectively [7].
References:
1. “Ramucirumab mCRC.” Food and Drug Administration. 24 Apr 2015. http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm444496.htm. 02 Jul 2015.
2. Nguyen, D.X., Bos, P.D., and Massague, J. “Metastasis: from dissemination to organ-specific colonization.” Nat Rev Cancer. 2009;9(4):274-84.
3. Bordeaux, J., Welsh, A.W., Agarwal, S., Killiam, E., Baquero, M.T., Hanna, J.A., Anagnostou, K., and Rimm, D.L. “Antibody validation.” Biotechniques. 2010;48(3).
4. Adams, G.P., and Weiner, L.M. “Monoclonal antibody therapy of cancer.” Nat Biotechnol. 2005;23(9):1147-57.
5. Bendardaf, R., Buhmeida, A., Hilska, M., Laato, M., Syrjanen, S., Syrjanen, K., Collan, Y., and Pyrhonen, S. “VEGF-1 expression in colorectal cancer is associated with disease localization, stage, and long-term disease-specific survival.” Anticancer Res. 2008;28(6B):3865-70.
6. “Receptor.” Dictionary.com. 2002. http://dictionary.reference.com/browse/receptor. 02 Jul 2015.
7. Tabernero, J., Yoshino, T., Cohn, A.L., Obermannova, R., Bodoky, G., Garcia-Carbonero, R., Ciuleanu, T., Portnoy, D.C., Van Cutsem, E., Grothey, A., Prausova, J., Garcia-Alfonso, P., Yamazaki, K., Clingan, P.R., Lonardi, S., Kim, T.W., Simms, L., Chang, S., and Nasroulah, F. “Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomized, double-blind, multicenter, phase 3 study.” Lancet Oncol. 2015; 16(5):499-508.
8. Javle, M., Smyth, E.C., and Chau, I. “Ramucirumab: successfully targeting angiogenesis in gastric cancer.” Clin Cancer Res. 2014;20(23):5875-81.
9. Panda, S., Zhang, J., Tan, N.S., Ho, B., and Ding, J.L. “Natural IgG antibodies provide innate protection against ficolin-optimized bacteria.” EMBO J. 2013;32(22):2905-2919.
10. Li, Z., Woo, C.J., Iglesias-Ussel, M.D., Ronai, D., and Scharff, M.D. “The generation of antibody diversity through somatic hypermutation and class switch recombination.” Genes Dev. 2004;18(1):1-11.
11. Malbec, O., and Daeron, M. “The mast cell IgG receptors and their roles in tissue inflammation.” Immunol Rev. 2007;217:206-21.
12. Fuchs, C.S., Tomasek, J., Yong, C.J., Dumitru, F., Passalacqua, R., Goswami, C., Safran, H., dos Santos, L.V., Aprile, G., Ferry, D.R., Melichar, B., Tehfe, M., Topuzov, E., Zalcberg, J.R., Chau, I., Campbell, W., Sivanandan, C., Pikiel, J., Koshiji, M., Hsu, Y., Liepa, A.M., Gao, L., Schwartz, J.D., and Tabernero, J. “Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial.” Lancet. 2014;383(9911):31-9.
- Cacciatore, P. “2011 ASCO: Additional Phase III Study Data Support the Potential Role of Avastin in Newly-Diagnosed & Recurrent Ovarian Cancer.” Libby’s H*O*P*E*. 09 Jun 2011. Photo from Genentech. https://healthinfoispower.files.wordpress.com/2011/06/angiogenesis-11.jpg?w=562. 02 Jul 2015.
- “How to Stop Metastasized Terminal Colon Cancer, Part 1.” 29 May 2010 http://www.herbalzym.com/wp-content/uploads/2010/05/colon-cancer.bmp. 02 Jul 2015.
1. “Ramucirumab mCRC.” Food and Drug Administration. 24 Apr 2015. http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm444496.htm. 02 Jul 2015.
2. Nguyen, D.X., Bos, P.D., and Massague, J. “Metastasis: from dissemination to organ-specific colonization.” Nat Rev Cancer. 2009;9(4):274-84.
3. Bordeaux, J., Welsh, A.W., Agarwal, S., Killiam, E., Baquero, M.T., Hanna, J.A., Anagnostou, K., and Rimm, D.L. “Antibody validation.” Biotechniques. 2010;48(3).
4. Adams, G.P., and Weiner, L.M. “Monoclonal antibody therapy of cancer.” Nat Biotechnol. 2005;23(9):1147-57.
5. Bendardaf, R., Buhmeida, A., Hilska, M., Laato, M., Syrjanen, S., Syrjanen, K., Collan, Y., and Pyrhonen, S. “VEGF-1 expression in colorectal cancer is associated with disease localization, stage, and long-term disease-specific survival.” Anticancer Res. 2008;28(6B):3865-70.
6. “Receptor.” Dictionary.com. 2002. http://dictionary.reference.com/browse/receptor. 02 Jul 2015.
7. Tabernero, J., Yoshino, T., Cohn, A.L., Obermannova, R., Bodoky, G., Garcia-Carbonero, R., Ciuleanu, T., Portnoy, D.C., Van Cutsem, E., Grothey, A., Prausova, J., Garcia-Alfonso, P., Yamazaki, K., Clingan, P.R., Lonardi, S., Kim, T.W., Simms, L., Chang, S., and Nasroulah, F. “Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomized, double-blind, multicenter, phase 3 study.” Lancet Oncol. 2015; 16(5):499-508.
8. Javle, M., Smyth, E.C., and Chau, I. “Ramucirumab: successfully targeting angiogenesis in gastric cancer.” Clin Cancer Res. 2014;20(23):5875-81.
9. Panda, S., Zhang, J., Tan, N.S., Ho, B., and Ding, J.L. “Natural IgG antibodies provide innate protection against ficolin-optimized bacteria.” EMBO J. 2013;32(22):2905-2919.
10. Li, Z., Woo, C.J., Iglesias-Ussel, M.D., Ronai, D., and Scharff, M.D. “The generation of antibody diversity through somatic hypermutation and class switch recombination.” Genes Dev. 2004;18(1):1-11.
11. Malbec, O., and Daeron, M. “The mast cell IgG receptors and their roles in tissue inflammation.” Immunol Rev. 2007;217:206-21.
12. Fuchs, C.S., Tomasek, J., Yong, C.J., Dumitru, F., Passalacqua, R., Goswami, C., Safran, H., dos Santos, L.V., Aprile, G., Ferry, D.R., Melichar, B., Tehfe, M., Topuzov, E., Zalcberg, J.R., Chau, I., Campbell, W., Sivanandan, C., Pikiel, J., Koshiji, M., Hsu, Y., Liepa, A.M., Gao, L., Schwartz, J.D., and Tabernero, J. “Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial.” Lancet. 2014;383(9911):31-9.
- Cacciatore, P. “2011 ASCO: Additional Phase III Study Data Support the Potential Role of Avastin in Newly-Diagnosed & Recurrent Ovarian Cancer.” Libby’s H*O*P*E*. 09 Jun 2011. Photo from Genentech. https://healthinfoispower.files.wordpress.com/2011/06/angiogenesis-11.jpg?w=562. 02 Jul 2015.
- “How to Stop Metastasized Terminal Colon Cancer, Part 1.” 29 May 2010 http://www.herbalzym.com/wp-content/uploads/2010/05/colon-cancer.bmp. 02 Jul 2015.
The Journal of Simplified Cancer Research (JSCR) is the official journal of Cancer Research Simplified. For only $4.99 per monthly issue, [or a discounted $49.99 per year], you can gain digital access to straightforward information and articles on cancer news, diagnosis, prevention, treatment, and clinical trials. Fill out the form, make your payment, and you'll receive your copy right in your inbox! To get your copy now on The Journal of Simplified Cancer Research (JSCR) page.










